Multiple Choice
If the accompanying drawing of an electrochemical cell represents an iron-nickel cell, use the partial activity series included to select the half-reaction represented by the RIGHT side of the cell. 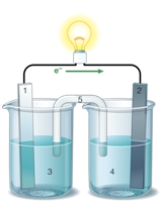 Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
A) Fe Fe2+ + 2e-
B) Ni Ni2+ + 2e-
C) Fe2+ + 2e- Fe
D) Ni2+ + 2e- Ni
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Which pair of half-reactions could be
Q14: Given the accompanying partial activity series,which
Q15: What are the coefficients of the
Q17: In the context of chemical reactions,oxidation refers
Q22: If the accompanying drawing of an electrochemical
Q25: Given the accompanying partial activity series,which
Q34: Considering the following net ionic equation:
Q39: Consider an electrochemical cell consisting of
Q48: Which example BEST represents the reaction
Q51: Given the accompanying partial activity series,which