Multiple Choice
The figure shows a pV diagram for 2.6 g of ideal helium gas that undergoes the process 1 → 2 → 3. Find the value of volume V3. The atomic mass of helium is 4.0 g/mol, and R = 8.31 J/mol ∙ K. 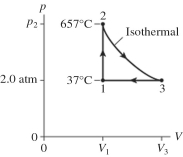
A) 25 L
B) 99 L
C) 50 L
D) 12 L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: When a gas expands adiabatically,<br>A)the internal (thermal)energy
Q19: How many grams of ice at
Q27: For an ideal gas,<br>A) <span
Q33: The melting point of aluminum is 660°C,its
Q41: A 771.0-kg copper bar is put into
Q42: A glass tea kettle containing 500 g
Q49: A 400-g block of iron at 400°C
Q55: An ideal gas is compressed isothermally to
Q91: A runner generates 1260 W of thermal
Q149: (a) At what Celsius temperature is the