Multiple Choice
The figure shows a pV diagram for 8.3 g of ideal nitrogen gas N2 in a sealed container. The temperature of state 1 is 59°C, the atomic mass of the nitrogen atom is 14 g/mol, and R = 8.31 J/mol ∙ K. What are (a) pressure p1 and (b) temperature T2? 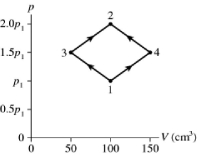
A) (a) 81 atm, (b) 660°C
B) (a) 14 atm, (b) 660°C
C) (a) 81 atm, (b) 120°C
D) (a) 14 atm, (b) 120°C
Correct Answer:

Verified
Correct Answer:
Verified
Q13: What is the average translational kinetic energy
Q22: In grinding a steel knife,the metal can
Q47: A rigid container is filled with
Q58: An aluminum electric tea kettle with a
Q59: A window glass that is 0.5 cm
Q77: A quantity of an ideal gas is
Q85: A sealed cylinder fitted with a movable
Q91: On a cold day,you take in 4.2
Q99: A blacksmith is flattening a steel plate
Q104: A thermally isolated system is made up