Multiple Choice
The figure shows a pV diagram for 2.9 g of ideal oxygen gas O2 in a sealed container. The temperature of state 1 is 76° C, the atomic mass of the oxygen atom is 16 g/mol, and R = 8.31 J/mol ∙ K. What are the temperatures T3 and T4? 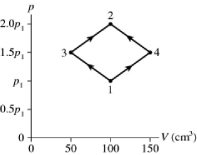
A) -11° C and 510° C
B) 57° C and 170° C
C) 260° C and 790° C
D) 38° C and 110° C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: When a sample of water at 0.0°C
Q37: How much heat is required to raise
Q39: A jogger is running outdoors on a
Q96: The thermal conductivity of a certain concrete
Q107: A giant star radiates energy at the
Q141: A lab assistant drops a 400.0-g
Q142: A steel pipe 36.0 m long,
Q143: A 0.40- <span class="ql-formula" data-value="m
Q148: In a flask, 114.0 g of
Q151: The temperature of an ideal gas