Short Answer
The figure shows a pV diagram of a gas for a complete cycle. During part bc of the cycle, 1190 J of heat flows into a system, and at the same time the system expands against a constant external pressure of as its volume increases from to Calculate the change in internal (thermal)energy of the system during part bc of the cycle. If the change is nonzero, be sure to indicate whether the change is positive or negative. 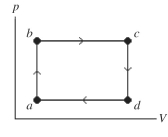
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Platinum melts at 3215°F.What is the corresponding
Q10: A 10-L flask and a 1-L flask
Q14: The temperature changes from 35°F during the
Q19: A heat pump absorbs heat from the
Q62: Express -40°C in °F.<br>A)-72°F<br>B)-54°F<br>C)-40°F<br>D)4.4°F
Q72: During each cycle, a heat engine takes
Q82: During each cycle,the compressor in a certain
Q83: A nuclear power plant has an
Q83: An important feature of the Carnot cycle
Q103: A temperature change of 20°C corresponds to