Multiple Choice
Refer to the following figure to answer the questions below.
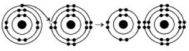
-When the atoms involved in a covalent bond have the same electronegativity, what type of bond results?
A) an ionic bond
B) a hydrogen bond
C) a nonpolar covalent bond
D) a polar covalent bond
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Which of the following are compounds?<br>A) H₂O,
Q10: Refer to the following figure to answer
Q11: Which bond or interaction would be difficult
Q12: When are atoms most stable?<br>A) when they
Q14: About 25 of the 92 natural elements
Q15: Compared with ³¹P, the radioactive isotope ³²P
Q16: Trace elements are those required by an
Q17: a(n. _ has charge but negligible mass,
Q18: Carbon-14 has the same _.<br>A) atomic number
Q41: Atoms have no electric charge because they