Multiple Choice
Use the following figure to answer the question.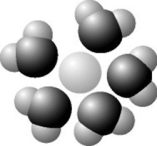
Based on your knowledge of the polarity of water molecules, the solute molecule depicted is most likely ________.
A) positively charged
B) negatively charged
C) without charge
D) nonpolar
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Consider two solutions: solution X has a
Q19: Liquid water _.<br>A) is less dense than
Q20: Hydrophobic substances such as vegetable oil are
Q21: The partial negative charge at one end
Q25: Why is a steam burn more severe
Q26: Measurements show that the pH of a
Q27: Sulfur is in the same column of
Q28: If the cytoplasm of a cell is
Q29: Which of the following is a hydrophobic
Q35: We can be sure that a mole