Multiple Choice
The solutions in the arms of a U-tube are separated at the bottom of the tube by a selectively permeable membrane. The membrane is permeable to sodium chloride but not to glucose. Side A is filled with a solution of 0.4 M glucose and 0.5 M sodium chloride (NaCl) , and side B is filled with a solution containing 0.8 M glucose and 0.4 M sodium chloride. Initially, the volume in both arms is the same.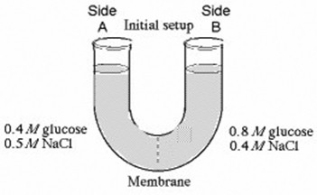
At the beginning of the U-tube experiment illustrated above, which of the following statements is true?
A) Side A is hypertonic to side B.
B) Side A is hypotonic to side B.
C) Side A is hypertonic to side B with respect to glucose.
D) Side A is hypotonic to side B with respect to NaCl.
Correct Answer:

Verified
Correct Answer:
Verified
Q39: The membranes of winter wheat are able
Q40: In which of the following environments would
Q41: What will happen to a red blood
Q42: The solutions in the two arms of
Q43: Why are lipids and proteins free to
Q45: Which of the following statements is most
Q46: The phosphate transport system in bacteria imports
Q47: What kinds of molecules pass through a
Q48: Five dialysis bags, constructed of a type
Q49: In what way do the membranes of