Multiple Choice
Use the following information to answer the question below.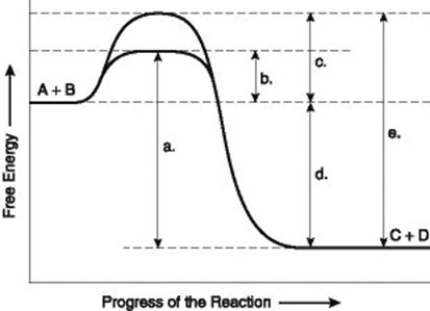
The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the following terms best describes the forward reaction in the figure?
A) endergonic, ∆G > 0
B) exergonic, ∆G < 0
C) endergonic, ∆G < 0
D) exergonic, ∆G > 0
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which of the following is an example
Q3: A decrease in entropy is associated with
Q4: _ is a regulatory mechanism in which
Q5: Use the following information to answer the
Q6: A series of enzymes catalyze the reactions
Q7: A series of enzymes catalyze the reactions
Q8: In a metabolic pathway, succinate dehydrogenase catalyzes
Q9: How might a change of one amino
Q10: Chemical equilibrium is relatively rare in living
Q11: Which of the following statements describes a