Multiple Choice
Which of the following graphs best depicts the entropy of a pure substance as the temperature is raised from its solid form through its liquid and gaseous forms?
A) 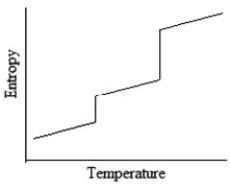
B) 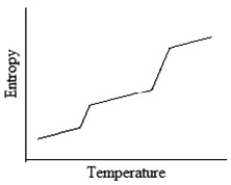
C) 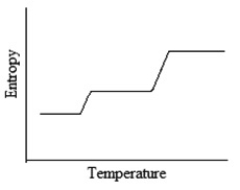
D) 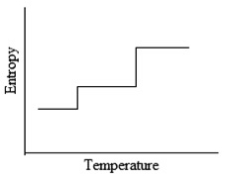
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q114: Which statement is true of the ideal
Q116: The standard molar enthalpy of fusion for
Q116: The standard molar enthalpy of fusion for
Q117: A reaction is at equilibrium at
Q118: Which statement characterizes the following table?<br>Temperature
Q120: Which statement characterizes the following table?<br>Temperature
Q121: Care must be taken when dissolving
Q122: In the equation relating equilibrium to
Q123: Which of the following must be
Q124: Which of the following is in the