Multiple Choice
A sketch of the free energy for a hypothetical chemical equilibrium is shown here. What part of the plot on the axis representing the relative quantities of reactants and products corresponds to a value of G that is greater than zero? 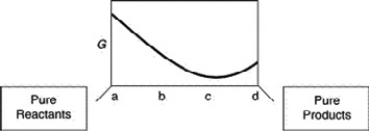
A) a to c
B) c only
C) c to d
D) b to d
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q57: Name and state the first three laws
Q80: What types of motion does a molecule
Q81: In an experiment, 1.00 mol of
Q83: Only one substance has a standard entropy
Q86: As the temperature of a reaction
Q87: Which of the following will have the
Q89: If 1 mol of ice melts at
Q90: As the temperature of an endothermic
Q123: Of the three modes of molecular motion-vibration,
Q127: The standard entropy of N<sub>2</sub>(g) is 191.5