Multiple Choice
A sketch of the free energy for a hypothetical chemical equilibrium is shown here. What part of the plot on the axis representing the relative quantities of reactants and products corresponds to a value of Q that is less than K? 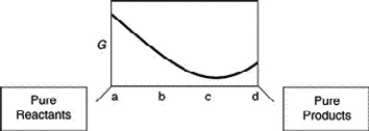
A) a to b
B) b to c
C) a to c
D) b to d
E) c to d
Correct Answer:

Verified
Correct Answer:
Verified
Q4: The equilibrium constant for a given reaction
Q81: Which of the following statements is/are correct?
Q122: In the equation relating equilibrium to
Q123: Which of the following must be
Q124: Which of the following is in the
Q125: Processes are always spontaneous when _
Q128: The following figures represent distributions of two
Q129: Several groups of general chemistry lab
Q131: Suppose <span class="ql-formula" data-value="\Delta"><span class="katex"><span class="katex-mathml"><math
Q132: Hydrogen reacts with nitrogen to form