Multiple Choice
What is indicated by the shape of the titration curve? 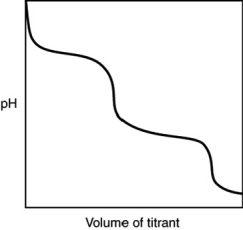
A) A diprotic acid was titrated with a strong base.
B) A triprotic acid was titrated with a strong base.
C) A diprotic base was titrated with a strong acid.
D) A triprotic base was titrated with a strong acid.
E) A strong acid was titrated with a strong base.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: How many moles of sodium acetate must
Q91: Lactic acid, which is found in milk
Q93: Define a conjugate acid-base pair and provide
Q94: Which expression defines the autoionization constant for
Q95: Phosphoric acid is a triprotic acid,
Q96: How would you calculate K<sub>b</sub> for the
Q97: Three common weak bases are phosphate (P;
Q100: Magnesium sulfate can be obtained at
Q102: When [H<sup>+</sup>] = 4.0 *10<sup>-</sup><sup>9</sup> M in
Q103: Which one of the following is a