Multiple Choice
A 0.500 g sample of an unknown substance was titrated with a 0.1 M HCl solution. Another 0.500 g sample of it was titrated with a 0.1 M NaOH solution. The resulting titration curves are illustrated here. Given the following possibilities, what is the sample? 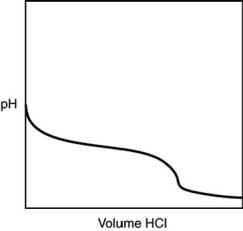
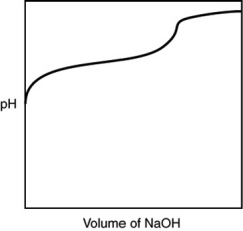
A) Na2CO3
B) NaHCO3
C) H2CO3
D) CO2
E) There is no way to tell.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: What is the pH of a solution
Q78: Which of the following compounds would not
Q158: The pK<sub>a</sub> of a weak acid was
Q159: To simulate the pH of blood, which
Q160: In the following reaction in aqueous
Q162: Halfway to the equivalence point in a
Q164: Pure water at any temperature has _<br>A)
Q166: Identify the weakest and strongest acids in
Q167: Bert and Ernie were determining the pH
Q168: To simulate the pH of blood, which