Multiple Choice
At what point in the following titration curve for a weak acid being titrated with a strong base is the pH equal to the pKa of the acid? The x-axis scale goes from 0.0 mL to 20.0 mL. The sharp rise is at 10.0 mL. 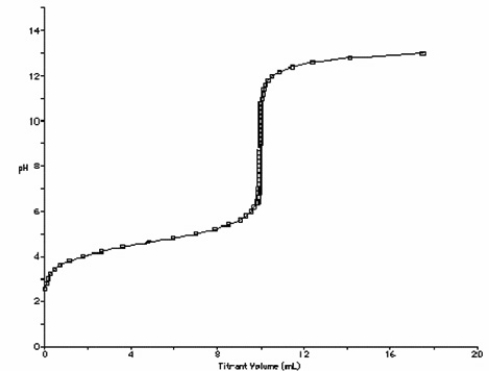
A) 0.0 mL
B) 5.0 mL
C) 9.0 mL
D) 10.0 mL
E) 18.0 mL
Correct Answer:

Verified
Correct Answer:
Verified
Q40: Write the reaction equation and the equilibrium
Q65: Write the reaction and equilibrium constant that
Q108: The hydronium ion concentration of a dilute
Q109: The solubility product for an insoluble salt
Q110: Define a Brønsted-Lowry acid and a Brønsted-Lowry
Q111: What is the pOH of a 0.20
Q113: Identify the weakest and strongest acids in
Q114: The Yucca Mountain repository in Nevada, which
Q115: Which statement, A-D, is not correct? If
Q116: Which sketch best represents the qualitative molecular