Multiple Choice
The XRD scan of the face-centered cubic (fcc) structure of sodium chloride showed that there was a distance of 562.8 pm between "layers of ions." Given that the fcc unit cell has a volume of 178.26 *10-24 cm3, to which distance does the 562.8 pm correspond?
A) 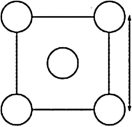
B) 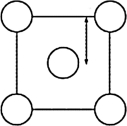
C) 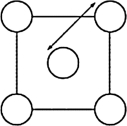
D) 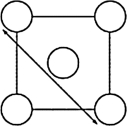
Correct Answer:

Verified
Correct Answer:
Verified
Q27: Which of the following unit cells has
Q79: Gold has a face-centered cubic structure with
Q84: An approximately spherical allotrope of carbon containing
Q93: In ionic solids, one ion often occupies
Q95: The alpha form of polonium (Po) has
Q98: Which of the following unit cells has
Q125: Why are ceramic superconductors not currently practical
Q126: Which of these is the best conductor
Q130: Firing of a kaolinite clay object to
Q134: Iron (Fe) crystallizes as a body-centered unit