Short Answer
Consider the three unit cells below for ionic solids. In each unit cell, one of the ions is arranged in one of the standard unit cell structures while the other is found between these sites. What are the names of the minerals associated with each of these structures? I ________; II ________;
III ________. 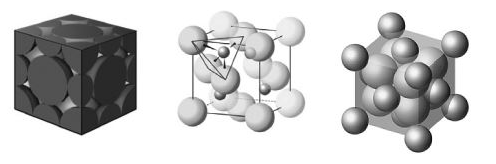
Correct Answer:

Verified
rock salt ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q14: A hexagonal closest-packed structure has hexagonally arranged
Q20: The closest-packing of spheres (such as oranges,
Q51: In the solid-state structure of sodium chloride,
Q84: An approximately spherical allotrope of carbon containing
Q93: In ionic solids, one ion often occupies
Q95: The alpha form of polonium (Po) has
Q109: Iron crystallizes in a body-centered cubic pattern.
Q130: Firing of a kaolinite clay object to
Q134: Iron (Fe) crystallizes as a body-centered unit
Q167: Which element would be used to dope