Multiple Choice
In the diagram below, one beaker contains pure water and the other contains an equal volume of seawater. Seawater has various salts dissolved in it. The beakers are sitting in a totally enclosed chamber, and the outside temperature and pressure are held constant. Identify the statements below about this situation that are not correct. 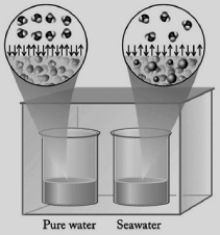 I. Water will be transferred from the pure water beaker to the seawater beaker.
I. Water will be transferred from the pure water beaker to the seawater beaker.
II) The vapor pressure of the seawater is higher than the vapor pressure of the pure water.
III) Pure water evaporates at a faster rate than seawater.
IV) Water in the gas phase condenses into the seawater beaker at a faster rate than into the pure water beaker.
A) I, III, and IV
B) II, III, and IV
C) II and III
D) II and IV
E) II only
Correct Answer:

Verified
Correct Answer:
Verified
Q14: Which one of the ionic compounds below
Q27: Which solution, if either, would create the
Q63: A 15.0 mg sample of a protein
Q64: What would be the freezing point of
Q66: Which statement A-D about ionic solids is
Q71: Use the following data to calculate
Q72: Ethylene glycol is used commonly as an
Q90: The term colligative refers to properties that
Q91: Which statement A-D about ionic solids is
Q120: The solubility of any gas in a