Multiple Choice
What is the vapor pressure of an aqueous solution that has a solute mol fraction of 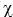 = 0.100? The vapor pressure of water is 25.756 mm Hg at 25°C.
= 0.100? The vapor pressure of water is 25.756 mm Hg at 25°C.
A) 23.2 mm Hg
B) 2.58 mm Hg
C) 25.8 mm Hg
D) 0.900 mm Hg
E) 22.3 mm Hg
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: Which intermolecular interactions are likely to result
Q20: Which statement below regarding vapor pressure is
Q23: Which of the solutions shown here will
Q27: The pressure of carbon dioxide in a
Q32: Determine the molal concentration of a sugar
Q36: Why would you expect or predict sodium
Q56: Indicate which aqueous solution has the highest
Q65: Which of the following compounds has the
Q137: You are working as an intern in
Q161: Which of the following gases do you