Multiple Choice
Which of the following diagrams best shows a set of polar molecules interacting through dipole-dipole interactions?
A) 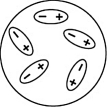
B) 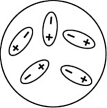
C) 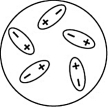
D) 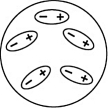
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Would water rise to the same height
Q82: Which hydride do you predict has the
Q83: CH<sub>2</sub>F<sub>2</sub> has a dipole moment of 1.93
Q84: Which of the following substances has a
Q85: Which is the dominant interaction between acetone
Q86: On the phase diagram below, identify the
Q88: Arrange the three ionic compounds sodium chloride,
Q89: The relative energies (strengths) of the intermolecular
Q153: Which is the dominant interaction that leads
Q160: Indicate which of the following pairs of