Multiple Choice
Which one of the following substances would you predict to have the highest vapor pressure at a given temperature? In these line drawings, a carbon is implicit at the end of a line (bond) or where two or more lines come together. Carbon requires four bonds, so any missing bonds are implicitly bonds to hydrogen atoms.
A) 
B) 
C) 
D) 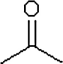
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Q25: Describe the similarity and the difference between
Q72: Ion-dipole forces always require_<br>A)an ion and a
Q81: A substance that is _ will be
Q101: Define the terms hydrophobic and hydrophilic.
Q119: In understanding why ammonia (NH<sub>3</sub>) has a
Q123: The smell of fresh-cut pine is due
Q124: The temperature at point a in the
Q125: Which one of the following substances would
Q127: On the phase diagram below, identify the
Q129: For each of the following pairs of