Multiple Choice
Which statement about the phase diagram below is not correct? 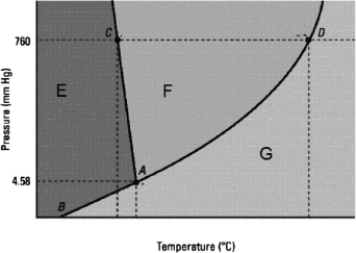
A) The critical point is not shown.
B) D is the normal boiling point.
C) The vapor pressure of the solid is zero below the triple point.
D) The normal melting point is at a lower temperature than the triple point.
E) E, F, and G label in that order the solid, liquid, and gas phases.
Correct Answer:

Verified
Correct Answer:
Verified
Q32: Which of the following substances is a
Q33: The relative energies (strengths) of the intermolecular
Q34: The density of water decreases as it
Q38: Gasoline is primarily a mixture of hydrocarbons
Q39: The relative energies (strengths) of the intermolecular
Q41: The interaction energy of LiF is -1.14
Q42: Which of the following compounds is capable
Q47: Dispersion forces are due to _<br>A)permanent dipoles.<br>B)temporary
Q82: A phase diagram shows the states of
Q138: Why do the strengths of dispersion interactions