Multiple Choice
At the point marked with a dot on the phase diagram, the solid will ________ 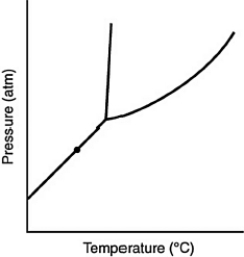
A) be indistinguishable from the gas.
B) boil.
C) melt.
D) sublime.
E) liquefy.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which of the following statements does not
Q15: What does the line indicated by the
Q17: Which hydride do you predict has the
Q18: Polarizability refers to _<br>A)the ease with which
Q18: Melting occurs in going from region _
Q19: Portable lanterns and stoves used for camping
Q27: Which of the following compounds would be
Q34: Predict which of the following ionic compounds
Q46: Explain why the boiling point of nitrogen
Q93: Which is the dominant interaction that explains