Multiple Choice
Consider the phase diagram for a substance shown here. The line between the solid and liquid phases has a positive slope because the solid phase is ________ than the liquid phase. 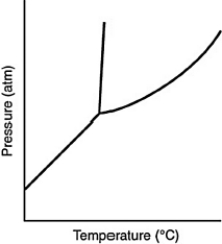
A) more dense
B) less dense
C) more massive
D) less massive
E) hotter
Correct Answer:

Verified
Correct Answer:
Verified
Q20: The vapor pressure of a liquid will
Q26: Gasoline is primarily a mixture of hydrocarbons
Q30: Point c in the phase diagram below
Q32: Which of the following substances is a
Q33: The relative energies (strengths) of the intermolecular
Q34: The density of water decreases as it
Q47: Dispersion forces are due to _<br>A)permanent dipoles.<br>B)temporary
Q82: A phase diagram shows the states of
Q84: Coulomb's law states that the energy of
Q119: A hydration sphere forms around an ion