Multiple Choice
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance has the highest melting point? 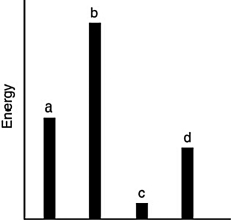
A) a
B) b
C) c
D) d
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which of the following statements does not
Q18: Melting occurs in going from region _
Q19: Portable lanterns and stoves used for camping
Q22: Arrange the following compounds in order of
Q26: Gasoline is primarily a mixture of hydrocarbons
Q27: Which of the following compounds would be
Q45: At the critical point, _<br>A)all the liquid
Q65: Describe the utility of the Clausius-Clapeyron equation.
Q84: Coulomb's law states that the energy of
Q119: A hydration sphere forms around an ion