Multiple Choice
Which of the substances a-d in the following figure has the weakest intermolecular forces? 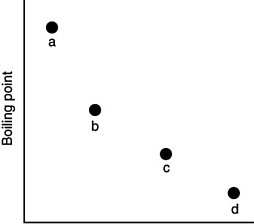
A) a
B) b
C) c
D) d
Correct Answer:

Verified
Correct Answer:
Verified
Q25: Describe the similarity and the difference between
Q44: Which molecule does not exhibit hydrogen bonding?<br>A)HF<br>B)CH<sub>3</sub>NH<sub>2</sub><br>C)CH<sub>3</sub>OH<br>D)(CH<sub>3</sub>)<sub>3</sub>N<br>E)CH<sub>3</sub>CH<sub>2</sub>OH
Q70: Which type of intermolecular interaction exists for
Q72: Ion-dipole forces always require_<br>A)an ion and a
Q81: A substance that is _ will be
Q112: CH<sub>2</sub>F<sub>2</sub> has a dipole moment of 1.93
Q113: Which of the following solvents will involve
Q115: The phase diagram for carbon dioxide
Q119: In understanding why ammonia (NH<sub>3</sub>) has a
Q129: For each of the following pairs of