Multiple Choice
What are the hybridizations of the carbon atoms in CH3CH2(CO) H, in order from left to right? Note that the 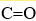 bond is a double bond.
bond is a double bond.
A) sp3, sp3, sp2
B) sp3, sp3, sp3
C) sp3, sp2, sp3
D) sp2, sp2, sp3
E) sp3, sp2, sp2
Correct Answer:

Verified
Correct Answer:
Verified
Q9: For the molecule CH<sub>3</sub>CHCHCH<sub>3</sub>, the local molecular
Q39: Both ethane (C<sub>2</sub>H<sub>6</sub>) and ethylene (C<sub>2</sub>H<sub>4</sub>) have
Q40: This diagram shows the structure of two
Q41: Of the following molecules (O<sub>3</sub>, SCl<sub>2</sub>, SO<sub>2</sub>,
Q43: Which electron-pair geometry corresponds to a steric
Q47: Which of the following molecules or ions
Q65: Which type of molecular orbital has only
Q116: In VSEPR theory, molecular geometry is determined
Q125: Which electron-pair geometry has the lowest electron-electron
Q153: Which of the following statements about bonds