Multiple Choice
Which one of the following molecules is paramagnetic? These molecules are described by the MO energy diagram below. These molecular orbitals are formed from the 2s and 2p atomic orbitals. 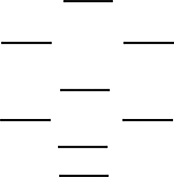
A) Li2
B) C22-
C) N22-
D) N22+
E) C2
Correct Answer:

Verified
Correct Answer:
Verified
Q81: Which electron-pair geometry corresponds to a steric
Q83: Which one of the following statements
Q85: Use energy levels of diatomic molecules derived
Q86: What is the valence electron molecular
Q87: What is the bond order of B<sub>2</sub>?<br>A)0<br>B)1<br>C)2<br>D)3<br>E)1.5
Q90: Using the energy-level diagram below, determine the
Q91: Ethanol has the formula CH<sub>3</sub>CH<sub>2</sub>OH. The oxygen
Q92: What is the valence electron molecular
Q101: Determine the molecular geometry of CF<sub>2</sub>Cl<sub>2</sub>.<br>A)linear<br>B)bent<br>C)trigonal bipyramidal<br>D)tetrahedral<br>E)trigonal
Q159: What is the molecular geometry of SF<sub>4</sub>?<br>A)tetrahedral<br>B)square