Multiple Choice
Boron nitride is being investigated in frontier research directed at producing novel electronic devices. If you used the following energy-level diagram for the molecular orbitals of boron nitride, BN, what would you predict? These molecular orbitals are formed from the 2s and 2p atomic orbitals. 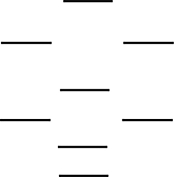 I. Boron nitride is diamagnetic.
I. Boron nitride is diamagnetic.
II) Boron nitride has a bond order of 2.
III) Boron nitride is paramagnetic.
IV) The bond in BN- is stronger than the bond in BN.
A) I and II
B) II and III
C) I, II, and IV
D) III
E) IV
Correct Answer:

Verified
Correct Answer:
Verified
Q7: Identify the hybridization of atomic orbitals for
Q8: Which of the following molecules or ions
Q9: Which of these molecules is chiral? <img
Q12: Which of the following ions is linear?<br>A)
Q13: Benzene (C<sub>6</sub>H<sub>6</sub>) is a cyclic, nonpolar molecule.
Q14: Which statement about VSEPR theory is not
Q104: All homonuclear diatomic molecules _<br>A)have polar bonds.<br>B)are
Q111: Which of the following molecules has a
Q127: Both diazene (N<sub>2</sub>H<sub>2</sub>) and hydrazine (N<sub>2</sub>H<sub>4</sub>) have
Q182: Draw a structure showing the geometry of