Essay
A Lewis structure of aspirin without the nonbonding electrons is shown in the figure below. Taking into account the nonbonding electrons, identify the hybridization of the atomic orbitals for the following atoms: O1, O2, and O3. Identify the bond angles around C1, C2, and O3. 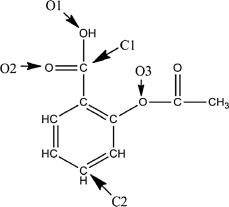
Correct Answer:

Verified
O1 is sp3 hybridized, O2 is sp2,...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: For which one of the following molecules
Q18: Which of the following molecules is paramagnetic?<br>A)Li<sub>2</sub><br>B)C<sub>2</sub><br>C)B<sub>2</sub><br>D)N<sub>2</sub><br>E)F<sub>2</sub>
Q96: Which type of molecular orbital is used
Q112: Which of the following compounds has a
Q120: Which type of molecular orbital contains a
Q126: Which of the following compounds has the
Q128: Which one of the following species has
Q132: Which statement about sigma and pi bonds
Q136: Which statement regarding a sigma bond between
Q140: The local molecular geometry and the hybridization