Essay
A Lewis structure of aspirin without the nonbonding electrons is shown in the figure below. Identify the hybridization of the C1 and C2 atomic orbitals. Arrange the bonds (B2-B5) in order of increasing length. 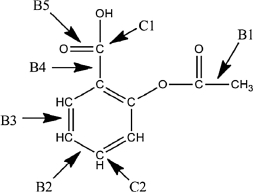
Correct Answer:

Verified
C1 and C2 are sp2 hybridized. B...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q21: Which statement A-D about VSEPR theory is
Q23: Which of the following has a central
Q25: Broccoli, cabbage, and kale contain compounds that
Q26: For the series methane, ammonia, and water,
Q28: Which bond is the most polar?<br>A) carbon-oxygen<br>B)
Q29: What is the valence electron molecular
Q30: The correct H-N-H bond angle in
Q32: What is the molecular geometry of the
Q122: Which statement A-D about valence bond theory
Q134: Which of the following molecules has a