Multiple Choice
Vinegar is a solution of acetic acid (C2H4O2) and water. Which of the following is the Lewis structure for acetic acid? 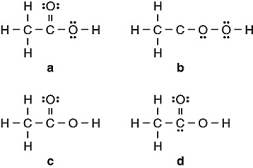
A) a
B) b
C) c
D) d
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: Explain why multiple Lewis structures are needed
Q96: Based on electronegativities, which bond of the
Q102: Which structure for dinitrogen sulfide (N _N
Q105: Which one of the following molecules or
Q107: The formal charge on the oxygen atoms
Q108: Which of the following has the most
Q109: Which one of the following Lewis symbols
Q110: Which of these statements correctly describes the
Q111: Which molecule has a stretching vibration that
Q139: How many valence electrons does S<sup>2</sup><sup>-</sup> have?<br>A)8<br>B)6<br>C)16<br>D)4<br>E)14