Multiple Choice
Electromagnetic radiation with a frequency of 8.6 *1014 Hz incident on an unknown metal surface causes ejection of photoelectrons with kinetic energies of 1.3 *10-19 J. What is the unknown metal?
A) rubidium (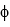 = 3.5 *10-19 J)
= 3.5 *10-19 J)
B) gold (11ef1a66_0479_eb58_a3cb_57e06677e6de_TB3834_11 = 8.2 *10-19 J)
C) nickel (11ef1a66_0479_eb58_a3cb_57e06677e6de_TB3834_11 = 8.3 *10-19 J)
D) sodium (11ef1a66_0479_eb58_a3cb_57e06677e6de_TB3834_11 = 4.4 *10-19 J)
E) platinum (11ef1a66_0479_eb58_a3cb_57e06677e6de_TB3834_11 = 9.1 *10-19 J)
Correct Answer:

Verified
Correct Answer:
Verified
Q11: How much energy is required to ionize
Q75: What is the wavelength ( <span
Q77: The energy of a one-electron atom
Q78: Write the electron configuration of Zn<sup>2+</sup>.
Q79: How much energy is required to ionize
Q81: A shell is defined by _<br>A)
Q82: Determine the wavelength of the line in
Q83: Which statement about the quantum numbers that
Q84: A light detector based on the photoelectric
Q85: What is the energy (E, in J)