Multiple Choice
What wavelength of light is required to cause the ejection of photoelectrons with kinetic energies of 5.0 *10-20 J from a calcium surface (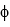 = 4.60 * 10-19 J) ?
= 4.60 * 10-19 J) ?
A) 485 nm
B) 390 nm
C) 308 nm
D) 632 nm
E) 480 nm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: Which of the following types of electromagnetic
Q15: Compared with the atomic radius of
Q16: Indicate which of the following sources
Q18: How many electrons can be in the
Q19: What color will a red object appear
Q20: Which of the following atoms or ions
Q21: Which prediction regarding the formation of a
Q22: Which transition in a hydrogen atom will
Q29: Atomic spectra are due to the changes
Q59: Arrange the following elements in order of