Essay
Consider the radial distribution shown below for an electron in an s atomic orbital where r is the distance of the electron from the nucleus. 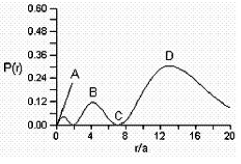
Identify: (1) where the atomic nucleus is located; (2) a radial node; (3) the most probable distance of the electron from the nucleus; and (4) the principle quantum number for this orbital.
Correct Answer:

Verified
(1) The nucleus is located at ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q97: What is meant when two or more
Q98: Fraunhofer lines are due to _<br>A) emission
Q99: Which subshell only has five orbitals?<br>A)
Q100: Astronomers have detected hydrogen atoms in interstellar
Q102: What is the energy (E, in
Q105: What are the principal and angular
Q106: Which statement A-D about the wave
Q107: What is the kinetic energy of the
Q108: If cesium, which has a work function
Q124: Which of the following electrons will have