Multiple Choice
The volume V (in liters) of a certain mass of gas is related to its pressure P (in millimeters of mercury) and its temperature T (in degrees Kelvin) by the law 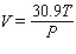 Compute
Compute 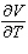 and
and 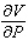 when T = 260 and P = 700.
when T = 260 and P = 700.
A) 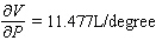 ;
; 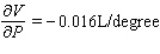 The volume increases by 0.044 L when the temperature increases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury.The volume increases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
The volume increases by 0.044 L when the temperature increases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury.The volume increases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
B) 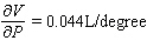 ;
; 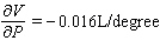 The volume increases by 0.044 L when the temperature increases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury.The volume decreases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
The volume increases by 0.044 L when the temperature increases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury.The volume decreases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
C) 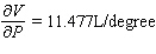 ;
; 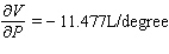 The volume increases by 0.044 L when the temperature decreases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury.The volume decreases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
The volume increases by 0.044 L when the temperature decreases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury.The volume decreases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
D) 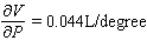 ;
; 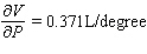 The volume increases by 0.044 L when the temperature increases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury.The volume increases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
The volume increases by 0.044 L when the temperature increases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury.The volume increases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
Correct Answer:

Verified
Correct Answer:
Verified
Q193: Use a double integral to find the
Q194: Evaluate the double integral
Q195: Evaluate the double integral <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7866/.jpg" alt="Evaluate
Q196: The projected number of wireless subscribers y
Q197: Find the equation of the least-squares line
Q199: Find the maximum and minimum values of
Q200: Find the critical point(s) of the function.Then
Q201: With an aging population, the demand for
Q202: Evaluate the double integral <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7866/.jpg" alt="Evaluate
Q203: According to industry sources, online banking is