Essay
What is the value of the equilibrium constant for the following equilibrium?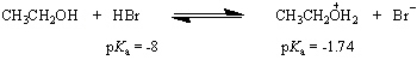
Correct Answer:

Verified
log10Keq = pKa(acid) - pK...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
log10Keq = pKa(acid) - pK...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q12: Which of the following compounds is the
Q29: Which species is the conjugate acid in
Q30: The pK<sub>a</sub> of acetic acid, CH<sub>3</sub>COOH, is
Q31: Complete the equation below for the protonation
Q32: The pK<sub>a</sub> of HCl is -7. What
Q35: Use curved arrows to show the movement
Q37: Which of the following statements is
Q63: Which of the following compounds is the
Q70: Which of the following has the highest
Q74: Which of the following compounds is the