Essay
What is the value of the equilibrium constant for the following equilibrium?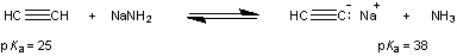
Correct Answer:

Verified
log10Keq = pKa(acid) - pK...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
log10Keq = pKa(acid) - pK...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q44: Which of the following equations is
Q45: Which of the following terms describes the
Q48: What is the value of the equilibrium
Q50: Provide the equation that relates the equilibrium
Q50: Which of the following has a pK<sub>a</sub>
Q52: Which of the following compounds has the
Q54: Which of the following is present in
Q59: What is the approximate pK<sub>a</sub> value of
Q65: Which of the following is a definition
Q89: Which of the following compounds has the