Multiple Choice
Which of the following resonance structures is the least important contributor to the resonance hybrid of the acetate anion, CH3COO-? 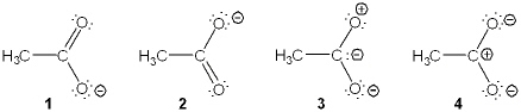
A) 1
B) 2
C) 3
D) 4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: Which of the following is a polar
Q24: Circle and name the functional groups in
Q25: Circle all of the sp hybridized atoms
Q28: Which of the following molecules has a
Q31: Circle all of the sp<sup>2</sup> hybridized atoms
Q32: What is the approximate C-C-C bond
Q74: What is the approximate strength of the
Q79: What is the approximate value of the
Q93: Draw bond-line structures of all of the
Q99: How many electrons are there in the