Multiple Choice
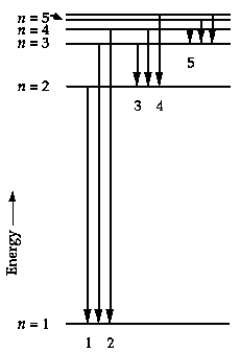 In the energy-level diagram, the line that corresponds to the longest wavelength in the Balmer series is
In the energy-level diagram, the line that corresponds to the longest wavelength in the Balmer series is
A) 1.
B) 2.
C) 3.
D) 4.
E) 5.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: The energy of a photon of visible
Q38: The symbol that represents the orbital angular
Q39: What is the momentum in SI units)
Q44: What is the energy difference between the
Q45: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7291/.jpg" alt=" This is an
Q48: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7291/.jpg" alt=" This is an
Q55: The velocity of escape of photoelectrons<br>A)increases with
Q94: J.J.Thomson's model of an atom<br>A)had electrons embedded
Q107: A photon of wavelength 80 nm is
Q134: A classical particle<br>A)can be localized.<br>B)can be scattered.<br>C)exchanges