Multiple Choice
The equation derived by Bohr for the wavelengths of λ of the lines in hydrogen- like spectra is 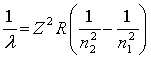 .The first member of the Balmer series of hydrogen has λ = 660 nm. Doubly ionized
.The first member of the Balmer series of hydrogen has λ = 660 nm. Doubly ionized 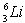 is hydrogen-like. The wavelength of the first member of the Balmer series for doubly ionized
is hydrogen-like. The wavelength of the first member of the Balmer series for doubly ionized 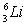 is
is
A) 73 nm.
B) 5.9 × 103 nm.
C) 150 nm.
D) 60 nm.
E) 1.8 × 10-3 nm.
Correct Answer:

Verified
Correct Answer:
Verified
Q10: The wavelength of red light is closest
Q48: The wavelength of blue light is closest
Q64: The ground-state energy of hydrogen is -13.6
Q74: Which of the following experiment(s)illustrates the particle
Q125: The energy of the n = 1
Q157: In a photoelectric experiment, the threshold frequency
Q158: In Young's experiment,<br>A) each slit acts as
Q161: A compact disc of a CD player
Q162: Which of the following formulas has the
Q166: 50 eV) when it is illuminated by