Multiple Choice
The energy of the nth level in a one-electron atom is 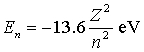 . Consider a beryllium ion with all but one of its electrons removed a beryllium atom normally has four electrons) . What is the energy of the electron when it is in the third-lowest energy state?
. Consider a beryllium ion with all but one of its electrons removed a beryllium atom normally has four electrons) . What is the energy of the electron when it is in the third-lowest energy state?
A) -24 eV
B) -7.6 eV
C) -1.5 eV
D) 24 eV
E) 7.6 eV
Correct Answer:

Verified
Correct Answer:
Verified
Q31: The kinetic energy of an electron moving
Q32: A gamma-ray photon of energy 100 keV
Q35: An electron m<sub>e</sub> = 9.11 × 10<sup>-31</sup>
Q37: A 10.0-kg mass moving with a velocity
Q38: The symbol that represents the orbital angular
Q39: What is the momentum in SI units)
Q47: The wavelength of an electron is 1.33
Q94: J.J.Thomson's model of an atom<br>A)had electrons embedded
Q102: An electron in a hydrogen atom jumps
Q107: A photon of wavelength 80 nm is