Multiple Choice
The energy of the nth level in a one-electron atom is 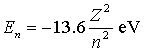 . Consider a beryllium ion with all but one of its electrons removed a beryllium atom normally has four electrons) . What is the wavelength of a photon emitted when the electron makes the transition from the third-lowest to the lowest energy state?
. Consider a beryllium ion with all but one of its electrons removed a beryllium atom normally has four electrons) . What is the wavelength of a photon emitted when the electron makes the transition from the third-lowest to the lowest energy state?
A) 1.03 × 10-7 m
B) 2.03 × 10-8 m
C) 6.43 × 10-9 m
D) 5.71 × 10-9 m
E) 1.03 × 10-27 m
Correct Answer:

Verified
Correct Answer:
Verified
Q5: A classical wave<br>A)behaves like a water wave.<br>B)exhibits
Q127: An electron in the hydrogen atom ground-state
Q129: The d state of an electronic configuration
Q130: What is the momentum in SI units)
Q131: Calculate the photon energy for light of
Q133: The first Bohr radius, a<sub>0</sub>, is 0.0529
Q134: The energy of a quantum of radiation
Q135: Magnetic resonance imaging MRI) is a much-used
Q136: The constant in the Rydberg formula is
Q137: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7291/.jpg" alt=" The figure shows