Multiple Choice
According to the Bohr theory, the allowed energy states for the hydrogen atom are given by the relation 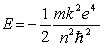 .This formula can be readily extended to other hydrogenic one-electron) systems. The energy of the second level n = 2) for the doubly ionized lithium atom is
.This formula can be readily extended to other hydrogenic one-electron) systems. The energy of the second level n = 2) for the doubly ionized lithium atom is
A) -54.4 eV.
B) 13.6 eV.
C) -30.6 eV.
D) -3.4 eV.
E) -1.5 eV.
Correct Answer:

Verified
Correct Answer:
Verified
Q35: Potassium has a work function of 2.3
Q48: The wavelength of blue light is closest
Q74: Which of the following experiment(s)illustrates the particle
Q151: In the Bohr model of the hydrogen
Q152: Bohr's quantum condition on electron orbits required<br>A)
Q153: When a certain x ray is Compton
Q154: The wavelength of the photon emitted when
Q157: In a photoelectric experiment, the threshold frequency
Q158: In Young's experiment,<br>A) each slit acts as
Q161: A compact disc of a CD player