Multiple Choice
For the principal quantum number n = 4, the number of values the orbital quantum number 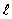 can have is
can have is
A) 4.
B) 3.
C) 7.
D) 16.
E) 6.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: The order-of-magnitude of the diameter of an
Q65: According to the Bohr theory,a hydrogen atom<br>A)does
Q92: An x-ray photon scattering off an electron
Q93: Rutherford's experiments, in which he bombarded a
Q95: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7291/.jpg" alt=" The set of
Q98: The possible orbital quantum numbers of an
Q101: The radius of the n = 1
Q102: 6 eV. The binding energy of the
Q130: A gamma-ray photon of energy 800 keV
Q140: An electron in the hydrogen atom makes