Multiple Choice
In the acid-base reaction between ammonia and water, which of the following substances are present at equilibrium? 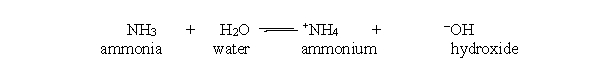
A) Ammonia, water, ammonium, and hydroxide are all present at equilibrium.
B) Only ammonia and water are present at equilibrium.
C) Only ammonium and hydroxide are present at equilibrium.
D) Only hydroxide and hydronium are present at equilibrium.
E) It is not possible to say which molecules will be present at equilibrium.
Correct Answer:

Verified
Correct Answer:
Verified
Q50: Which of the following compounds is a
Q87: Which of the following statements about bases
Q88: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution
Q90: Which of the following is NOT a
Q91: All acid-base reactions that we consider in
Q93: Which of the statements best describes the
Q94: How do strong and weak acids differ?<br>A)
Q95: Which of the following molecules is amphoteric?<br>A)
Q96: The boxed species in the following reaction
Q97: Metabolic acidosis is a type of acid-base