Multiple Choice
The following figure illustrates the action of a HF and F buffer where the sizes of the boxes are proportional to the concentrations of HF and F in solution. Which of the following chemical equations represents the reaction that occurs when -OH is added to the HF/F buffer? 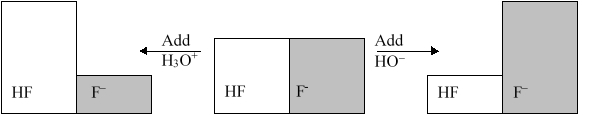
A) HF + H2O  F + H3O+
F + H3O+
B) F + H2O  F + OH
F + OH
C) HF + OH  F + H2O
F + H2O
D) F + 2 OH  HF + O2
HF + O2
E) F + OH  HOF
HOF
Correct Answer:

Verified
Correct Answer:
Verified
Q94: How do strong and weak acids differ?<br>A)
Q95: Which of the following molecules is amphoteric?<br>A)
Q96: The boxed species in the following reaction
Q97: Metabolic acidosis is a type of acid-base
Q98: Which of the following molecules is a
Q100: At physiological pH, phosphate esters, such as
Q101: Which of the following strong acids is
Q102: The concentration of H<sub>3</sub>O<sup>+</sup> in a solution
Q103: An alkene has a pK<sub>a</sub> of 40,
Q104: According to the chart below, how are