Multiple Choice
The buffering system of the blood is shown below. Which of the following equations represents the reaction that occurs when hydroxide is added to this buffer? 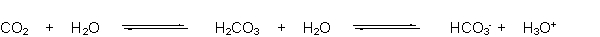
A) CO2 + OH → CO32 + H+
B) H2CO3 + OH → HCO3 + H2O
C) HCO3 + OH → CO32 + H2O
D) HCO3 + OH → CO2 + H2O2
E) H3O+ + OH → 2 H2O
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Which of the statements describes the following
Q21: Ammonia and ammonium are both present in
Q22: Each circle is a sample of an
Q23: Which of the following statements does NOT
Q24: Where does digestion of proteins begin?<br>A) in
Q26: Which of the following statements best describes
Q27: Which of the following statements about acids
Q28: The following reaction is a reversible reaction.
Q29: Methylamide (CH<sub>3</sub>CONH<sub>2</sub>) has a K<sub>a</sub> of 1
Q30: A sample of gastric juice has a