Multiple Choice
When a person hyperventilates, they breathe very quickly, exhaling CO2 more quickly than it can be produced. This reduces the concentration of CO2 in the blood. According to the chemical equation below and LeChatelier's principle, how is the bicarbonate equilibrium affected by the reduction of CO2 in the blood? 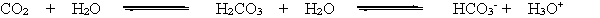
A) The equilibrium is unchanged.
B) More H3O+ is produced.
C) The equilibrium is shifted to the right.
D) The H3O+ concentration decreases.
E) More HCO3 is produced.
Correct Answer:

Verified
Correct Answer:
Verified
Q3: A conjugate acid-base pair is<br>A) the reactants
Q75: Lidocaine, a common injectable dental anesthetic, is
Q76: In the body, ethylene glycol (antifreeze) is
Q78: Which of the following reactions best illustrates
Q79: Which of the following is a balanced
Q79: The neutralization reaction of potassium hydrogen carbonate
Q81: Which of the following changes in blood
Q82: Select the choice that correctly states whether
Q84: Which of the following would be the
Q85: Not all protons in a molecule can