Multiple Choice
Consider the two containers below, which are separated by a semipermeable membrane that only allows the passage of small solutes. If A contains 6% NaCl in water and B contains 3% NaCl in water, which of the following will occur? 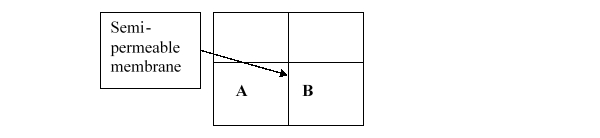
A) The volume of water in A will increase.
B) The volume of water in B will increase.
C) NaCl will flow from A to B.
D) NaCl will flow from B to A.
E) Nothing will happen.
Correct Answer:

Verified
Correct Answer:
Verified
Q17: Which of the following does NOT occur
Q26: Which of the following statements explains why
Q29: The concentration of an adrenalin chloride solution,
Q30: How many moles of magnesium chloride (MgCl<sub>2</sub>)
Q32: A 1% epinephrine solution is delivered by
Q33: How many grams of dextrose are in
Q35: What volume of a 6.0% solution of
Q36: According to a blood test, a patient
Q42: Which container would be the best for
Q62: Cephadrine is one of many antibiotics that